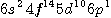원자이름 |
탈륨 |
원자량(Atomic weight) |
204.3833 |
전자 배치(Electron Configuration) |
[Xe] |
원자 반지름(Atomic Radius) |
2.08 |
녹는점(Melting Point)[1 atm] |
304.00° |
끓는점(Boiling Point)[1 atm] |
1473.00° |
융해열(Heat of Fusion) |
4.14kJ/mol |
기화열(Heat of Vaporization) |
n/a |
전기 음성도(Electronegativity) |
2.04 |
1st Ionization Potential |
6.108V |
2nd Ionization Potential |
20.428V |
3rd Ionization Potential |
29.830V |
밀도(Density) |
11.85g/cm |
비열(specific heat) |
0.13J/gK |